 |
| Visit our sponsor at www.chemglass.com |
 |
| Visit our sponsor at www.chemglass.com |
 |   |
| Reductive Elimination |
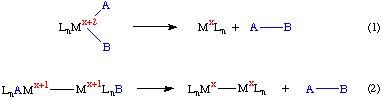
Reductive elimination is formally the microscopic reverse of oxidative addition, and it is not surprising that a series of reactions involving an oxidative addition, a rearrangement and then a reductive elimination form the basis for a variety of industrially important catalytic cycles.
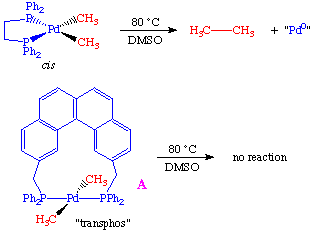
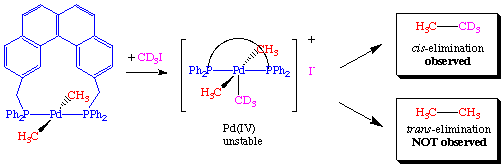
To rule out the possibility that the transphos ligand was simply changing the chemistry, the authors performed a crossover experiment which further supported the hypothesis of a mutually cis requirement:
This latter reaction is also an example of an oxidatively induced reductive elimination (see below).
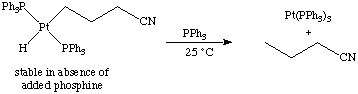
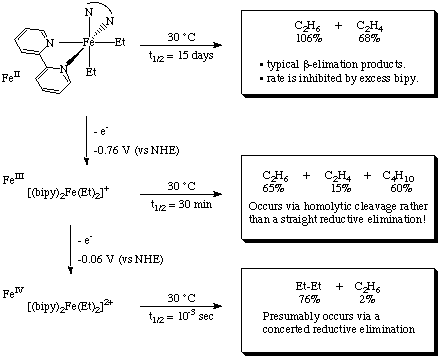
Notice that three different decomposition mechanisms are operative for the three different oxidation states of iron!
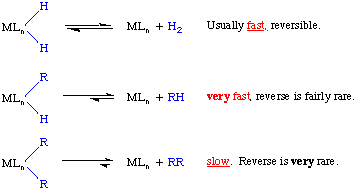
If we consider that the DH-H = 104 kcal/mol and that the DM-H is 50-60 kcal/mol we see that these are essentially balanced and there should be no thermodynamic preference for a dihydride versus a reduced metal center.
But DR-H is typically 100 kcal/mol versus a metal alkyl bond strength of 30 to 40 kcal/mol. We see that the thermodynamic situation is again approximately balanced with a slight preference for the forward reaction.
DR-R is typically around 90 kcal/mol, so for two alkyl substituents, there is a strong thermodynamic driving force for the reaction to go to the right. C-C bond activation is unusually rare, but more examples continue to be found.
| If you like the self-test exercises presented here, give me some feedback via email. If there is enough support, I'll add more throughout the OMHTB. - RT |

[Index] [Keyword Search] [Books & Software] [ILPI Home Page]
Please visit our sponsor to thank them for supporting this site!
This page was last updated Tuesday, March 31, 2015
This document and associated figures are copyright 1996-2025 by Rob Toreki or the contributing author (if any) noted above. Send comments, kudos and suggestions to us by email. All rights reserved.