Bubblers are simple devices used to maintain an inert atmosphere over a reaction apparatus while also providing for a means for pressure relief. Bubblers are typically filled with mercury or mineral oil, however the latter is recommended because mercury bubblers splash quite a bit and pose
a toxicity hazard. Shown here is the simplest possible setup involving a bubbler:
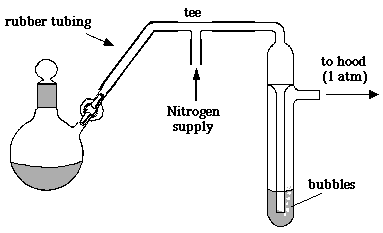
When the pressure inside your apparatus is greater than the laboratory's atmospheric pressure, excess gas will bubble down the tube and out through the mineral oil. If the pressure inside your apparatus falls below atmospheric pressure, oil will rise in the tube and prevent air from entering the system. However, if the pressure is too low, air will eventually get in and you will suck oil (or mercury) into your apparatus. This is the kind of mistake you generally make only once or twice (the tedious cleanup is a great learning experience).


 The Glassware Gallery
The Glassware Gallery
![]()